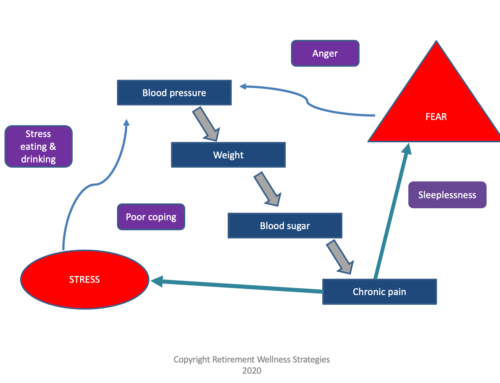
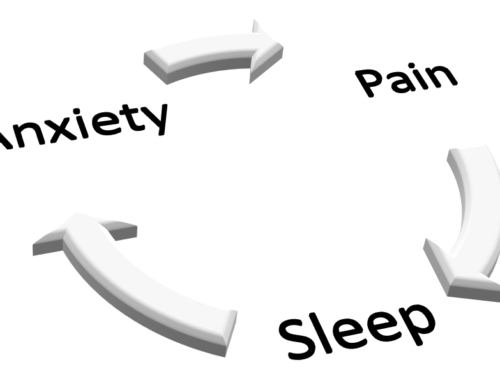
All natural doesn’t necessarily mean safe and useful. Know before you decide so you can make informed decisions.
Years ago I started having a discussion with patients who were making personal decisions about their health. “If you reach the last days of your life and say, ‘Yes, this is what I expected’, then I have done my job informing you of your options.” “If you reach your final days and say, ‘If I had known this I would have made different decisions’, then I have not done my job.”
As a clinical pharmacist and a professor, educating is one of the primary roles I have.
Personal Health Decisions
I am now frequently talking with clients about the substances they choose to take on their own. There are so many options! These can be anything from vitamins to supplements to over-the-counter medicine to naturopathic substances to homeopathic substances to formerly illicit now legal substances to still illicit substances.
It is now rare to encounter a client who is not taking at least one self-selected substance.
My first question is always why a substance was selected and what was the decision making process used to choose it. Was it a fully informed decision?
Research Pros and Cons
The most common answer is that the client has read a lot and carefully researched the substance. There is usually also a component of recommendation from a friend or other user of the substance.
Doing your research is a GREAT thing! I encourage you to read from a variety of sources.
There seems to be an undercurrent of distrust in Western medicine. Certainly the Pharmaceutical Researchers and Manufacturers of America (PhRMA) has done several things to earn the distrust from pricing to availability to the actions of individual companies and their products. [The example that jumps to mind right now is Purdue Pharma and their questionable practices with Oxycontin®.]
Too often in every segment of society, one bad apple can taint the whole bushel. To many people, anything touting a ‘natural’ source and not within the control of PhRMA seems to be the safer and healthier choice.
Evidence-Based Medicine
A big purpose of this blog is to define evidence-based medicine (EBM) and explain the differences from many of the other substance-based therapies you can choose. The ‘evidence’ comes from well-designed studies that measure both whether the medication works and the safety. Further, it helps to define how much better is it than other known options, what doses work best in what sorts of people, and which side effects are minor and which are major.
Medications approved by the Food and Drug Administration (FDA) must have well-designed studies that demonstrate both efficacy (does it work) and safety. Then, the FDA assures any company making the medication is making it in a clean facility using precise methods assuring each individual dose is per these high standards.
Two examples of the critical nature of EBM that comes to my mind are with heart medicines and with hormone replacement in women. The Cardiac Arrhythmia Suppression Trial (CAST) was the first in my career to completely change practice. It had long been assumed that using medication to keep the heart rhythm normal after a cardiac procedure would be a good thing. Then, this well-designed study demonstrated that in many instances, suppressing the abnormal heart rhythms right after a procedure actually increased risk of death. That was completely unexpected! But, other studies have confirmed that these findings. So, many of the medications that used to be part of standard treatment are no longer even made anymore.
The other big example was use of hormone replacement in women near and beyond menopause. The assumption was that keeping hormone levels where they are during the fertile years would maintain health and prevent many health issues that come after menopause. Then, two well-designed studies demonstrated that assumption was completely wrong! The dangers of hormone replacement were much greater than any advantages. Premarin ® and Prempro ® went from some of the most commonly prescribed medications to very rarely used.
Substances without this level of evidence
The FDA does not oversee the vast majority of the substances you can choose on your own. (The noted difference is the over-the-counter medications that are overseen by the FDA.) So, unless the company does this on its own, no one is overseeing accuracy of the label, the contents, the conditions of the manufacturing facility, or the consistency from dose to dose.
In addition, there is no requirement for well-designed studies to define when, how, and at what dose the substance is most effective or what are the side effects.
Even medication interactions with your other medications is not well known since this type of data is not required.
Healthcare providers and self-treatment
Many of my clients are disappointed that their doctors aren’t more supportive of their self-selected therapies. Some don’t even tell their doctor about these.
I HIGHLY encourage you to share ALL substances you take with each of your doctors and your pharmacist. Healthcare providers have been trained to care for you using evidence-based medicine. There is too little ‘data’ to give providers confidence with other therapies. Your doctor can’t provide you with assurance of either if the substance will work for you or if it will be safe for you. Communicate all you know to help make informed decisions.
Purpose of this blog – make informed decisions
I encourage you to share your research, the reason you take the substance, and anything you’ve noticed that is different since starting the substance with your healthcare team. Then, work together to decide all of the substances you will take. Each member of your team needs to known the full picture in order to take the best care of you.
This will also allow you to make the most informed decision possible. Combine your research, your personal goals, and your doctor’s and pharmacist’s input in your decision. Then, monitor your response. In your final days, know that you made the best decisions you could make for your own health allow the way.
Contact me
If you would like to discuss these concepts or have personal input from a board certified specialist, give me a call at 410-472-5078 or e-mail me at michelle@retirewellness.com. Check out my website at www.retirewellness.com.
For further application, check out my personal blog.